- Home >
- Short-tailed Albatross >
- Future Activities –Reintroduction
Short-tailed Albatross
Reintroduction to Ogasawara Islands
Update: June 30, 2017
The Yamashina Institute for Ornithology has been continuing conservation activities of the endangered Short-tailed Albatross in Torishima of Izu Island chain. The success of the Project Decoy at Hatsunezaki in Torishima in the breeding season of 2005-6 led to the decision that the institute will continue the monitoring of individuals in Torishima but in addition begin a reintroduction project in Ogasawara Islands in order to recover the Short-tailed Albatross population to a safer state. Since 2005 candidate locations have been selected and many other preparation work have been conducted. This page explains the background and the present status of the project.
Problems faced by the existing two breeding sites
Among the existing two breeding sites of Torishima and Senkaku Islands, Torishima is much larger and there are approximately 1655 individuals as of May 2005 according to Hasegawa's estimation. The each of the breeding sites faces issues concerning conservation. Torishima is a volcanic island thus its biggest issue is that the breeding site is always faced with the risk of eruption. If a large eruption occurs during the breeding season much of the breeding population can be lost. On the other hand, it is difficult to conduct studies and conservation activities in Senkaku Islands. Due to these backgrounds, the importance of establishing a third breeding site for enhancing reliability of the population recovery has long been called for among those involved in conservation of the Short-tailed Albatross.
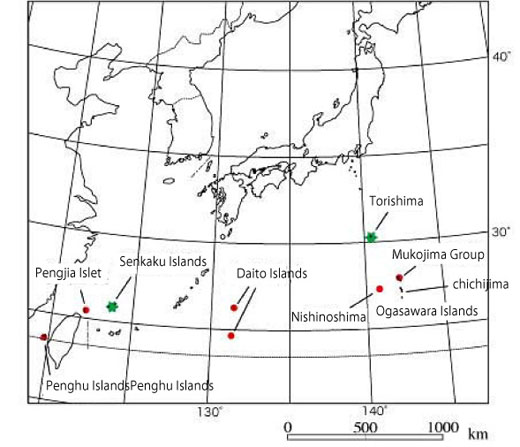
Island where the Short-tailed Albatross used to breed.
Before the over-hunting in the Meiji era there were breeding areas all over the northwestern Pacific (red dots) besides the present two breeding sites (green stars)
(Created based on Hasegawa 1995)
Making Ogasawara the third breeding site
Discussion among relevant parties including those in charge of wildlife conservation in the US government and researchers interested in the recovery of the Short-tailed Albatross as national endangered species of the US led to launching of the project to establish the third breeding site of the Short-tailed Albatross in Mukojima of the Ogasawara Island chain. Mukojima is located approximately 350km to the south-south-east of Torishima, the largest breeding site of the Short-tailed Albatross. This site was chosen as the candidate for a new breeding site because;
1) Mukojima group used to be a breeding site of Short-tailed Albatross before the over-hunting in Meiji-era and a small number of individuals have been currently observed landing there,
2) there is no danger of volcanic eruption,
3) relative species, Black-footed and Layan Albatross breed on Mukojima. They have a potential to attract Short-tailed Albatross.
4) Chichijima, the center of the Ogasawara Islands, can be used as the base for conservation activities and for procurement of food and supplies.
How to attract Short-tailed Albatross – transplanting chicks, artificial rearing
In order to attract Short-tailed Albatrosses in Mukojima, the decoys and sound playback proved to be effective at Hatsunezaki in Torishima was used. However, this method required almost 15 years to establish a breeding site even though it was within the same island. A new method being considered for implementation in Mukojima involves transporting chicks hatched in Torishima and making them fledge from Mukojima. The Short-tailed Albatross is said to remember the site it was raised, and return to the site several years later when they are ready to breed themselves. This project tries to artificially rear chicks at the new site over a certain period of time and fledge them from there so as to increase the number of albatrosses returning to the site for breeding in a shorter time.
Artificial rearing of chicks in an uninhabited island will require refrigerators and a minimum level of living facilities, thus require extensive preliminary studies and preparation. As for transporting chicks, it was being considered to test with Black-footed Albatross, closely related to Short-tailed Albatross but under less risk of extinction before conducting it directly with the Short-tailed Albatross.
As for the project site, there were several candidate sites as Mukojima group is currently uninhabited and there are numerous existing breeding sites of seabirds. The site needs to be selected based on several criteria, such as present and current distribution of albatrosses, ease of berthing for transportation of goods and installation of accommodation facilities. The possible candidates being considered were Yomejima, Nakodojima and Mukojima.
Understanding and cooperation by local people are indispensable
Ogasawara Islands is designated as a national park and has many restrictions for conservation such as prohibition of camping and building of artificial structures. In order to protect the endemic species from alien species, transportation of goods from an island to island is also under strict inspection. Furthermore, vegetation of Mukojima islands is significantly damaged by feral goats and the Tokyo Metropolitan Government is currently leading a vegetation recovery project. Moreover, consideration for local industries such as tourism and fisheries is also important. Thus, this project will be promoted by overcoming legislative and regulatory issues and by ensuring sufficient communication with relevant stakeholders of the local government, fisheries, tourism, and nature conservation.

Mukojima group has the breeding sites of closely related Black-footed Albatrosses (March 2005 in Yomejima)

Flat area in the northwest of Mukojima, one of the candidate sites
Preparation for the next step
In March 2005 a staff from the Yamashina Institute for Ornithology together with Hiroshi Hasegawa (professor of Toho University) visited Ogasawara Islands and selected the northwestern part of Mukojima as the candidate site for the project. Following this, an environmental impact assessment was conducted by experts of various biological species and the results indicated that the implementation of the project does not have major problems. Furthermore, the institute organized several explanatory meetings in Chichijima and Hahajima in order to obtain understanding of the local people who are highly concerned with the local nature conservation.
After such preparatory work, the 30 Short-tailed Albatross decoys were installed at the project site located in the northwestern part of Mukojima.
As for artificial rearing of the transplanted chicks, a trial experiment has been conducted using a closely related species in order to clear out any problems that may occur. First of all, experimental rearing of Laysan Albatross Phoebastria immutabilis chicks was conducted in Kauai Island in Hawaii from March to July 2006. Ten chicks were transported from Midway Island and were artificially reared, and four of them successfully fledged. Among the six others, five died of infectious disease and bad weather and one is being reared at the Monterey Bay Aquarium as it cannot fly (*). Taking account of the lessons learned by this experimental rearing, second trial of Black-footed Albatrosses was conducted in Ogasawara Islands from March to June 2007. Ten chicks transported from Nakodojima to Mukojima were artificially reared and nine of them successfully fledged. Good results were obtained indicating that compared to the chicks reared by wild adult birds, the artificially reared chicks were no significant different in body sizes evaluated by multiple indices, and their body weight at the time of fledging was even a little heavier.
Based on these results, the project obtained the approval of the "Short-tailed Albatross Subcommittee of the Wildlife Conservation Measures Committee" of the Ministry of the Environment and it was decided that ten chicks of about 40 days old are transported from Tsubamezaki breeding site in Torishima and are artificially reared in Mukojima.
The Short-tailed Albatross becomes about 1.5 times bigger than the Laysan Albatross or the Black-footed Albatross experimentally reared, and they are much more sensitive to human presence. Thus it became an even bigger challenge. The researchers were working hard to successfully raise the chicks for the further recovery of the population of the Short-tailed Albatross.
(Click to see the bigger image)
(*) The details regarding the experimental rearing of the Laysan Albtross can be found in Harada, T., et al. 2008. An Artificial Rearing Experiment of Laysan Albatross Chicks. Journal of the Yamashina Institute for Ornithology, 39(2): 87-100.
Translocation and Hand-rearing of Short-tailed Albatross
Each February during 2008-2012, 10-15 Short-tailed Albatross chicks (30-40 days old) were captured and translocated from the main colony, Tsubame-zaki, on Torishima to Mukojima (Fig. 1a). In total 70 chicks were translocated: 31 female and 39 male. Different nests at Torishima were selected each year to prevent individual pairs experiencing apparent breeding failure more than once.
The chicks were placed in individual ventilated wooden boxes (40 x 40 x 50 cm) with sides lined with sponges against shock to prevent injury during transit and an absorbent sheet on the bottom (Fig. 1b). Box lids were fitted with thermometers to monitor internal box temperature. We opened the ventilation hatch when the internal box temperature exceeded 35°C.
Based on the results of Black-footed Albatross translocation, we decided to transport Short-tailed Albatross chicks by helicopter rather than by boat, despite the additional cost. To reduce the risk of helicopter/albatross collisions on Torishima, the boxes were carried by field personnel to the top of the 120-m high Tsubame-zaki, where they were loaded onto a helicopter and transported for 90 minutes (350 km) south to Mukojima. Upon arrival at the helicopter landing site, chicks were carried 1 km to the release site. As before, we removed chicks from their boxes and placed them 5–10 m apart on flat ground devoid of natural nest depressions. Total transit time was 6–8 hours.
On the day following release, a numbered plastic identification band (Y01–Y74) was placed on the left leg of each chick. Scheduling of Short-tailed Albatross chick translocations was also adjusted according to weather forecasts for the source colony and release site.

Figure 1. (a) The release site on a headland on the west end of Mukojima where Black-footed and Short-tailed Albatross chicks were hand-reared in 2007–2010.

Figure 1. (b) Short-tailed Albatross chick placed in a ventilated and temperature-monitored wooden box with sides lined with sponges and an absorbent sheet on the bottom.
During the first 2–5 days following translocation, 80–156 g of pureed therapeutic pet food (Prescription Diet a/dTM) and 300 ml of lactic Ringer’s solution or physiological salt solution, diluted twice by spring water, were administered daily to facilitate recovery from the stress of moving. Thereafter, we presumed the birds had recovered from this stress, and they were subsequently given darkedged-wing flying fish, Japanese common squid and canned oil sardine or Japanese sardine, plus Pacific krill. Short-tailed Albatross chicks were given 300–900 g of a mixture of these foods daily according to their daily metabolized energy per unit body mass (840 kJ kg-1) estimated from related species and the energy density of each food.
This feeding regime was continued until chicks were about 100 days old (mid-April), when chick body mass was at its maximum. We then gradually reduced the amount of food by 50–66% to decrease chick body mass by 20–30% prior to fledging. We administered the pureed foods and probiotics for the first 16–30 days, and then gave chopped or whole foods for the following 69–72 days. We also administered appropriate amounts of vitamin complex tablets (Mazuri Vita-Zu Bird TabTM, Mazuri Auklet VitaminTM). We gave 300–450 ml liquid containing 95% spring water and 5% clean seawater daily. For all chicks, feeding commenced on the day following release. Although feeding was continued until fledging to increase their post-fledging survival, amounts were limited to 100–300 g food and 30–300 ml of liquid every two or three days for 2–3 weeks prior to anticipated fledging.
There foods were kept cool in an insulated bin until arrival at the hand-rearing site and were heated in 50°C water just before feeding. The therapeutic pet food and pureed food was administered using 350 ml cartridges and caulking guns (Fig. 2a) through a silicon tube. We used 450 ml syringes fitted with a silicon tube to give liquid. Short-tailed Albatross chicks were always restrained with fleece blankets and handled by two persons to prevent injury during feeding. To minimize stress, handlers approached Short-tailed Albatross chicks in single file. We maintained high levels of hygiene by using disposable gloves for preparing meals and feeding activities. We sterilized all feeding equipment using 70% ethanol and no equipment that contacted chicks was reused without being sterilized. Used equipment was soaked in soapy and chlorine solutions recommended for baby bottles overnight.

Figure 2. (a) Hand-feeding of translocated Short-tailed Albatross chick (50–60 days of age) using a caulking gun. Chicks were restrained with fleece blankets to prevent injury during handling.

Figure 2. (b) Translocated Short-tailed Albatross chick (100-110 days of age) fed a whole squid.
Fledging success of hand-reared was 99% overall (69 of 79) during 5 years translocation. Growth of hand-reared chicks was comparable to that of naturally reared chicks, although hand-reared chicks were slightly larger at fledging (Fig. 3). Hand-reared birds had similar or better overall health than naturally reared birds, based on analysis of blood chemistry health indices, although the analysis indicated possible muscle stress in hand-reared birds (elevated levels of aspartate aminotransferase and creatine kinase). The techniques developed in our studies have broad-reaching implications for the future conservation of threatened populations of other surface-nesting seabirds. For more detailed information see Deguchi et al. (2012, 2014).
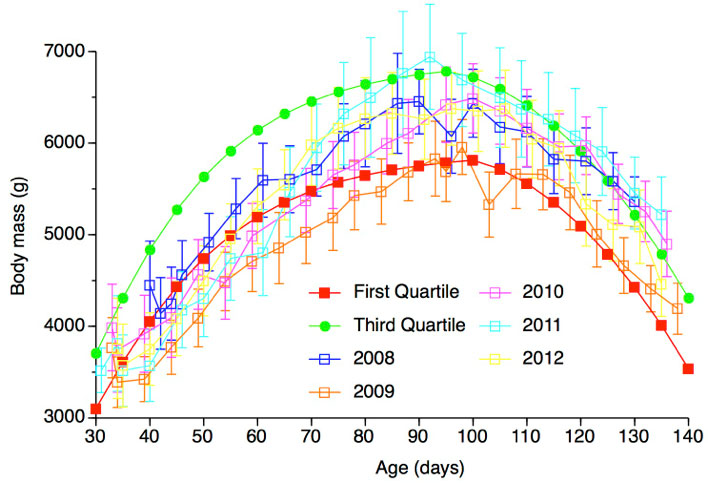
Figure 3. Growth trajectory of naturally reared chicks on Torishima (first and third quartile of body mass) and 2008-2012 hand-reared chicks on Mukojima (mean body mass ± SD).
Attendance and breeding attempts of Short-tailed Albatross in Ogasawara Islands
Restoration or establishment of colonies using translocation and hand-rearing can be an effective tool for conserving birds. However, well-designed post-release evaluation studies for long-lived species are rarely implemented. We investigated the attendance and breeding attempts of hand-reared Short-tailed Albatross chicks (n = 69) translocated to a historic breeding island in the Ogasawara Islands, 350 km from the source colony, for 8 consecutive years after the first translocation.
We observed Short-tailed Albatross visiting the translocation site while we hand-reared chicks from 08:00 to 11:00 every 1–3 days from February through May 2008–2012 (mean: 26 days per month). From November through March 2012–2016, for 4–15 days per month (mean: 8 days per month), we monitored and individually identified Short-tailed Albatross visiting Mukojima. We observed birds during 07:00 to 16:00 from a 30-m high cliff, 300-m from the translocation site, using 8–10x binoculars and a 60x spotting scope.
We also monitored a new colony on Torishima for 10–28 days (mean: 17 days) in February–March 2009–2016. This colony is a gathering place of almost all young, newly recruiting Short-tailed Albatross to Torishima because the main colony has little space available for new recruits. Observations were conducted on the gentle slope 80 m away from the colony between 07:00 and 17:00 using 8–10x binoculars and 60x spotting scope. Additionally, we obtained information of potential breeding sites other than Mukojima throughout the Ogasawara Islands annually monitored by Tokyo Metropolitan Government and the Institute of Boninology.
The number of hand-reared Short-tailed Albatross returning each year was lower at Mukojima versus Torishma (Fig. 1). Although, naturally reared Short-tailed Albatross had also begun visiting Mukojima since the translocation project began (Fig. 2). Twenty-seven of the 69 (39%) hand-reared Short-tailed Albatrosses returned to Mukojima at least once per breeding season. Twenty-six of the 69 (38%) hand-reared Short-tailed Albatrosses visited Torishima, of which 18 visited both islands. Hand-reared Short-tailed Albatross began returning to both Mukojima and Torishima 3 year after the first translocation (Fig. 2). There were no differences in sex ratio or body sizes at fledging among birds returning to either location versus non-returning birds.
The first breeding attempts by hand-reared Short-tailed Albatross occurred at Mukojima, the translocation site, and Torishima, the natal island, 5 years after the first translocation. The pair on Mukojima comprised of a 2008 hand-reared male (Y01) from Torishima and a naturally reared female from Senkaku Islands incubated an egg during three consecutive breeding seasons without hatching a chick. The eggs were suspected to be either infertile or the embryo died. Finally during their fourth season as a pair, a chick hatched and successfully fledged.
Additionally, a pre-fledging Short-tailed Albatross chick was incidentally found on Nakoudojima, 5 km south from Mukojima, 6 years after the first translocation. A pair comprised of a female hand-reared in 2009 (Y11) and a naturally reared male that was ringed at the natal colony on Torishima was found at the nest site on Nakoudojima the next season, but no chick was present. A third chick reared in the Ogasawara Islands was found on Yomejima (27.50°N, 142.21°E, 22 km south from Mukojima) during the eighth year after translocation. This near fledging age nestling was found during an annual survey of the islands and it was unknown whether either of the parents was hand-reared or both naturally reared.
By 8 year after the first translocation, seven hand-reared and two unidentified Short-tailed Albatrosses had successfully paired and fledged 12 chicks, three pairs at Mukojima and neighboring islands (three chicks total) and five pairs at Torishima (nine chicks total). In all known cases, the hand-reared Short-tailed Albatrosses paired with naturally reared birds, except one unidentified pair (Yomejima), and all but one naturally reared bird was from Torishima.
Our preliminary results suggest that even though more translocated and hand-reared albatrosses visited and recruited to their natal island compared to the translocation site, the early re-establishment of breeding by short-tailed albatrosses in the Ogasawara Islands 80 years after extirpation would not have occurred without the initial translocation effort. Further study is needed, however, to fully understand formation of breeding colonies beyond conspecific attraction and philopatry. For more detailed information see Deguchi et al. (2017).
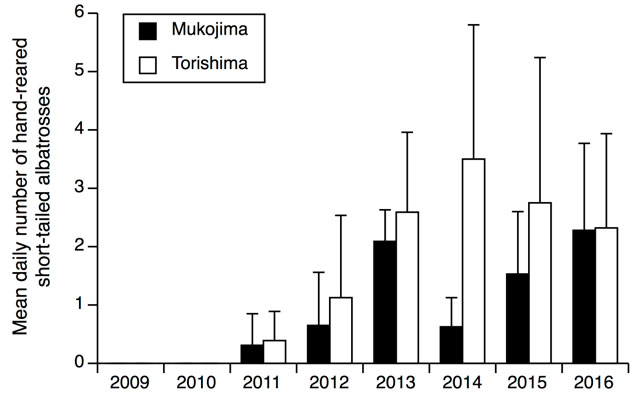
Figure 1. Mean (+ SD) daily number of hand-reared short-tailed albatrosses observed at the Mukojima translocation site vs. Torishima, the source island, during February–March 8 year post translocation.
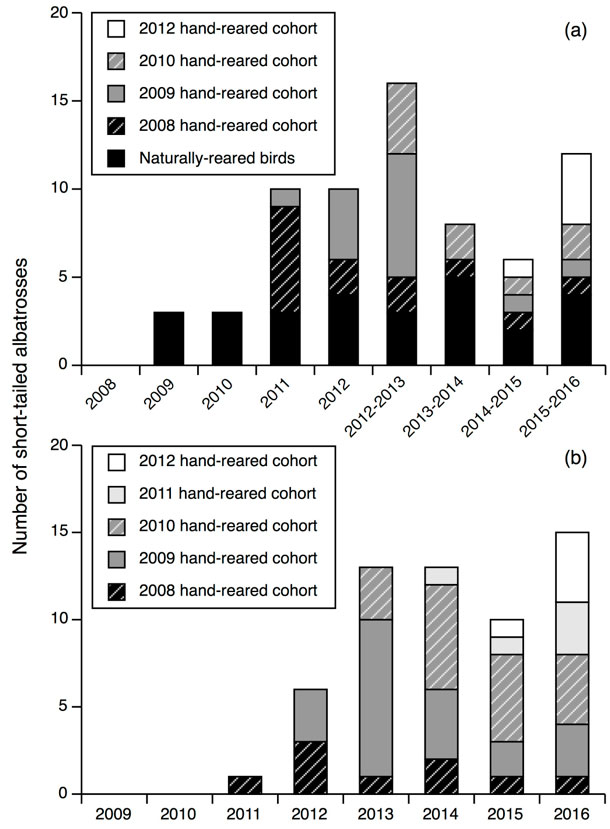
Figure 2. Annual number of unique individuals by cohort observed at the Mukojima translocation site (a) vs. Torishima (b), the source island, during 8 year post translocation. 2011 hand-reared cohort was not observed at Mukojima.
Post-fledging survival and migration
Post-fledging survival and migration were studied by satellite tracking a sample (40–50% annually) of hand-reared chicks on Mukojima and an equal number of naturally-reared chicks on Torishima (n = 5, 2008; n = 7, 2009; n = 6, 2010; n = 7, 2011; n = 6, 2012). Hand-reared chicks were selected for tagging based on sex and development (i.e. closest to fledging) and naturally reared chicks were selected opportunistically and based on development from different sections of the main colony each year. We used 22 g (< 1% of body mass) solar-powered Argos/GPS PTT-100 satellite transmitters (Microwave Telemetry, Columbia, USA). These devices recorded the geographical coordinates of six locations per day (at 2–4 hour intervals) and transmitted these locations every 3 days. Locations were recorded at 2-hour intervals (n = 46 transmitters) during 07.00–17.00, with a 12-hour off-duty cycle. Positional accuracy was < 10 m. Tracking devices were attached in May, 1–19 days before fledging. Devices were either taped to the back-feathers (n = 46, deployment duration < 6 months, Supplementary Plate 1g) or attached by a custom harness of double-layered tubular Teflon ribbon (n = 16, deployment duration < 3 years). The global positioning system (GPS) was essential for determining when chicks first went to sea, accurately calculating movement rates for analysis of post-fledging behavior, and estimating survivorship.
Tracking data indicated that 20 km h-1 (mean over a 2–4 hour period) was a threshold preceding sustained flight, whereas birds that did not survive showed primarily passive drifting at speeds < 5 km h-1. After leaving the colony fledglings typically drifted at sea for a mean of 9 days (range = 2–21 days), with only short flights, before attaining sustained flight. There was no significant difference in the number of days to sustained flight between male hand-reared and naturally reared fledglings. Mean post-fledging survivorship to sustained flight was 85% and was not significantly different between hand-reared and naturally reared chicks or transmitter attachment method. There was one hand-reared and one naturally reared fledgling mortality in 2008, two naturally reared fledgling mortalities each year in 2009–2010, three hand-reared fledgling mortalities in 2011 and no immediate post-fledging mortalities in 2012. During their first 6 months (the maximum attachment period for tape-attached transmitters), fledglings ranged widely throughout the North Pacific rim, with some also spending time in oceanic waters between Hawaii and Alaska (Fig. 1). The percentage overlap of the at-sea kernel density distributions of hand-reared and naturally reared birds was 74% for 95% kernel home ranges and 58% for 50% kernel core use areas. During the first 6 months of tracking there was no significant difference between hand-reared and naturally reared birds in transmitter deployment duration, total distance travelled or distance travelled per day. The results to date, however, have exceeded initial expectations and can inform potential reintroductions of other long-lived, migratory avian species with strong natal philopatry, and reintroductions of native species to former breeding islands. For more detailed information see Deguchi et al. (2014).
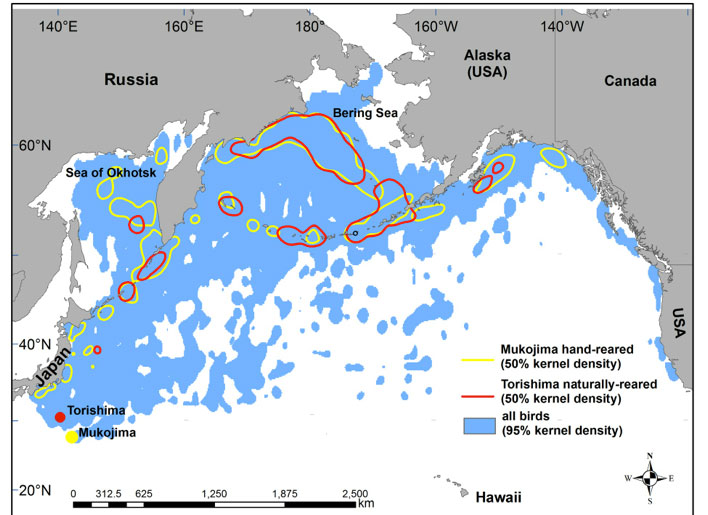
Figure 1. Post-fledging dispersal of hand-reared and naturally reared Short-tailed Albatross chicks during 6 months (May–November) post fledging, showing the 95% kernel density range of all birds and the 50% kernel density contours for hand-reared and naturally reared birds. Distribution data were obtained from Argos-linked GPS tracking of 27 hand-reared (3,220 bird-days) and 26 naturally reared (3,001 bird-days) individuals.

